
Several treatment challenges exist in the management of Ph+ ALL1-5
Explore considerations for adults newly diagnosed with Ph+ ALL
Challenges exist when treating adult patients newly diagnosed with Ph+ ALL1-5
Lack of consensus in frontline treatment
TKIs are part of guideline-recommended treatment regimens1 ; however, none had been FDA-approved.
Aggressive disease with high mutation burden
• Patients with Ph+ ALL have a high probability of developing mutations in BCR::ABL12,3
• Mutations lead to resistance and are a primary cause of relapse after 1st- and 2nd-generation TKIs4
Failure to achieve a complete molecular response
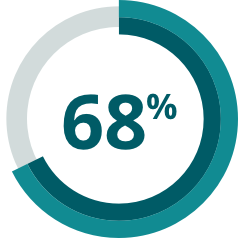
- In a meta-analysis, over 2/3 of patients failed to achieve a complete molecular response with 1st- or 2nd-generation TKIs + chemotherapy5*
- Outcomes with 1st- and 2nd-generation TKIs vary by choice of agent and chemotherapy regimen3
MRD-negative CR is a stringent composite endpoint combining both hematologic and molecular response1,8
MRD negativity8
Molecular assessment
≤0.01% BCR::ABL1 or undetectable BCR::ABL1 transcripts in cDNA with ≥10,000 ABL1 transcripts
- In ALL, MRD negativity is recognized as an important prognostic indicator of clinical outcomes across genetic subtypes6
- Although MRD negativity is a measure of cancer on the molecular level, National Comprehensive Cancer Network® (NCCN®) recommends adequate count recovery per protocol before transitioning to post-remission therapy for patients who achieve it1,7
Complete remission1*
Hematologic assessment
Patient meets all of the following for at least 4 weeks:
• No circulating blasts and <5% blasts in the bone marrow
• Normal maturation of all cellular components in the bone marrow
• No extramedullary disease
• ANC ≥1000/mcL
• Platelets ≥100,000/mcL
*Complete remission is also known as complete response.
Redefining remission in
1st-line Ph+ ALL
Comparable safety profile to imatinib in a clinical trial
ANC=absolute neutrophil count; cDNA=complementary DNA; CR=complete remission; mcL=microliter; MRD=minimal residual disease; NCCN=National Comprehensive Cancer Network; Ph+ ALL=Philadelphia chromosome-positive acute lymphoblastic leukemia; TKI=tyrosine kinase inhibitor.